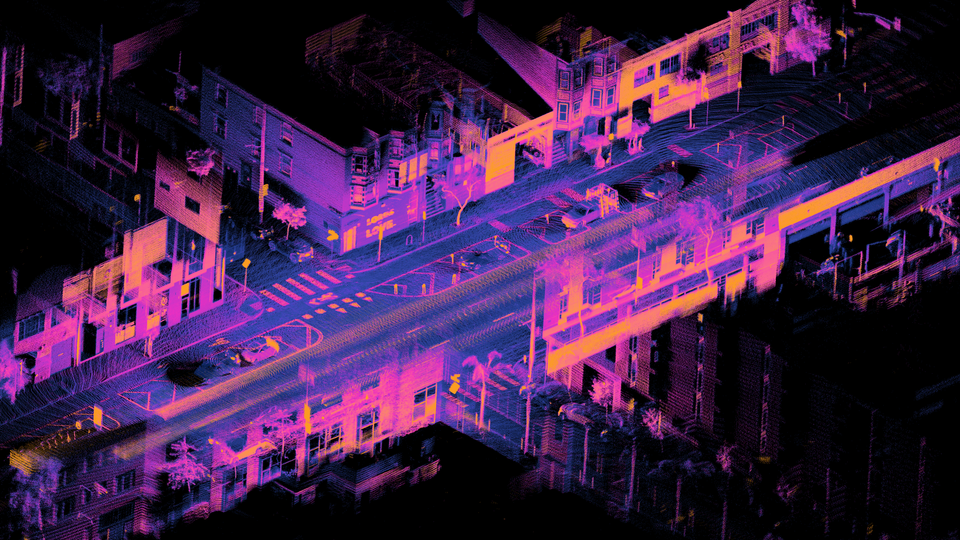
Ouster: Building The Interfaces Between AI And The Physical World
Ouster is positioned to provide essential perception interfaces that allow AI to act within the physical world, as digital LiDAR might become a primary sensory layer for autonomous systems.