Computational Modeling Of Palisade Bio's PALI-2108 Tissue Penetration In Fibrostenotic Crohn's Disease
PALI-2108's Phase 1b data for ulcerative colitis are promising, but UC is mucosal while FSCD is transmural. A computational reaction-diffusion model explores whether PALI-2108 can penetrate Crohn's strictures deeply enough to intercept fibrosis early.

Disclosure: The author holds a beneficial long position in Palisade Bio, Inc. (NASDAQ: PALI). This article is provided for informational and educational purposes only and is not financial advice. The reaction-diffusion model presented here is a speculative theoretical exercise built on estimated parameters and class-level pharmacology, not direct measurements of PALI-0008 in strictured tissue. It cannot reliably predict the outcome of any real-world study. Although the author is a Medical Doctor, this content represents a personal analytical perspective on the company's science and does not constitute medical advice, a diagnosis, or a treatment recommendation. The author receives no compensation for this article and has no business relationship with the company mentioned. Please see the full "Legal Information and Disclosures" section below.
Up to 70% of Crohn's patients eventually require surgery for fibrostenotic complications, and the cumulative risk of developing stricturing or penetrating disease reaches approximately 50% at 20 years after diagnosis. There are currently no approved anti-fibrotic drugs for intestinal strictures. Any drug that could slow the progression from early to advanced strictures would address one of the largest unmet needs in gastroenterology.
PDE4 inhibition has a credible anti-fibrotic rationale: cAMP suppresses TGF-beta/Smad signaling, inhibits myofibroblast differentiation, and reduces collagen synthesis. Nerandomilast, a PDE4B inhibitor for idiopathic pulmonary fibrosis, demonstrated stabilization of lung function in Phase 2 with probability of superiority over placebo of 0.998. The problem is that systemic PDE4 inhibitors (rolipram, apremilast, roflumilast) were all limited by intolerable nausea from PDE4D inhibition in the brainstem.
If PDE4 inhibition can affect fibrotic pathways, the question becomes how to deliver it to the right tissue without intolerable side effects. This is where Palisade Bio enters the picture. The company spent years developing LB1148, a serine protease inhibitor for postoperative GI recovery, before licensing Giiant Pharma's prodrug platform in September 2023 and pivoting into inflammatory bowel disease. The deal brought GT-2108 (renamed PALI-2108) for ulcerative colitis (UC) and fibrostenotic Crohn's disease (FSCD), plus GT-1908 for ileal Crohn's. PALI-2108 incorporates a galactose-derived sugar moiety cleaved by bacterial beta-glucuronidase in the ileocolonic lumen, releasing the active metabolite PALI-0008 locally with a colon-to-plasma AUC ratio exceeding 200.
In May 2025, Phase 1a data in healthy volunteers showed PALI-0008 was detectable in colonic tissue for at least 36 hours after a single dose, at concentrations near or above the IC90 for PDE4B. The IC90 is the drug concentration needed to inhibit 90% of the target enzyme's activity, and it serves throughout this analysis as the threshold above which the drug is considered pharmacologically effective. PK modeling from the Phase 1a data showed that the 36-hour tissue half-life supports once-daily dosing.
By August 2025, the Phase 1b ulcerative colitis cohort reported a clinical response in all five patients treated with 30 mg twice daily for seven days. The observed effects included a mean 63% reduction in modified Mayo score, one clinical remission, 51% reduction in tissue PDE4B expression, increased mucosal cAMP in four of five patients, 40% decrease in tissue lymphocytes, and RNAseq-confirmed downregulation of inflammatory, fibrotic, and companion diagnostic biomarkers, all with no serious adverse events.
These results in five patients should be interpreted carefully though. Five of five successes allows a one-sided 95% Clopper-Pearson lower confidence bound of 54.9% for the true response rate (via the Beta(5,1) quantile function), excluding a true response rate below that threshold at the 5% significance level. But it remains an uncontrolled observation in a disease with 25 to 40% placebo response rates and high symptom variability. Even a genuine drug effect cannot be separated from regression to the mean. The consistency across orthogonal pharmacodynamic endpoints (protein, cellular, transcriptomic) makes a pure placebo explanation less likely but does not exclude it, because all endpoints were measured in the same five patients, so a single placebo responder improving across correlated biomarkers could drive concordance.
The Phase 1b UC results were promising, but UC is a mucosal disease while FSCD is transmural, meaning it affects the whole bowel wall. In October 2025 Palisade Bio dosed the first patient in its Phase 1b of PALI-2108 for FSCD, using a once-daily regimen rather than the twice-daily schedule used in the UC cohort. This raises a question that clinical data alone cannot yet answer: can a luminally released drug physically reach the fibroblasts that drive stricture formation deep within the bowel wall?
One important caveat limits what the existing clinical data can tell us: standard colonoscopic forceps biopsies sample only the mucosa, typically reaching 0.55 to 0.66 mm deep. All of Palisade's reported tissue pharmacodynamics come from this shallow zone. They confirm potent local activity where drug concentration is highest but tell us nothing about deeper tissue.
That deeper tissue is exactly where fibrostenotic Crohn's disease lives. Ulcerative colitis is a mucosal disease: inflammation stays within the top 0.5 to 0.8 mm. FSCD is transmural. Ultrasound studies report wall thickness of 7.3 mm in naive terminal ileal strictures and 9.3 mm in established strictures, with the worst conglomerate masses exceeding 15 mm. The UC data prove the drug works where it is present in abundance. The question is whether PALI-0008 can penetrate deeply enough into a thickened, fibrotic bowel wall to suppress the signaling that drives stricture formation, and whether treating patients early could prevent the wall from thickening beyond the drug's reach.
To explore this, we built a speculative reaction-diffusion model in Python. It is important to be upfront: this is a theoretical exercise, not a clinical prediction. The model relies on estimated parameters, published morphometry from resection specimens, and class-level pharmacology rather than direct measurements of PALI-0008 in strictured tissue. It can suggest plausible ranges and identify which variables matter most, but it cannot predict what will happen in a real-world study. Every number that follows should be read with that caveat firmly in mind.
The model first needs to know how the bowel wall restructures itself in FSCD. Zhang and colleagues (Hum Pathol, 2018) performed digital morphometry on ileal stricture resection specimens, directly applicable to the FSCD trial's ileal biopsies. They found that the muscularis mucosae, normally just 0.05 mm thick in formalin-fixed sections, expands 17.7-fold in severe strictures, while the inner muscularis propria expands only 1.9-fold and the outer 1.3-fold. Chen and colleagues (J Crohns Colitis, 2017) confirmed that smooth muscle hyperplasia dominates, with obliterative muscularization replacing the normally loose submucosa. These morphometric data come from hypertrophic strictures, the classic form with concentrically thickened walls. Liu and colleagues (Clin Gastroenterol Hepatol, 2022) identified a distinct constrictive phenotype with reduced external circumference and little mural thickening. Those constrictive strictures would not follow the expansion model used here.
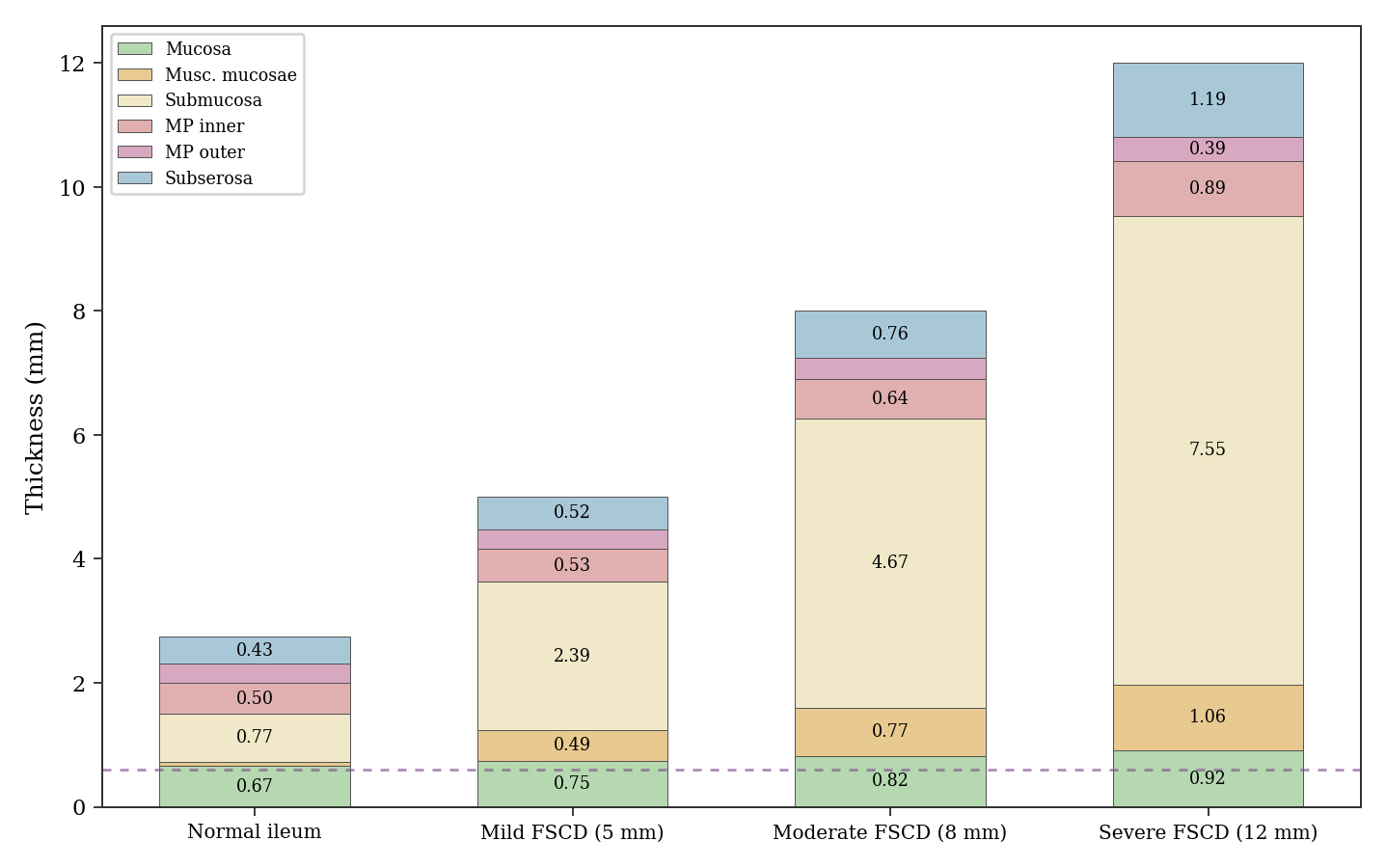
The resulting architecture is shown in Fig. 1. The normal wall, corrected for formalin shrinkage with layer-specific factors ranging from 1.25x to 1.54x (yielding approximately 2.7 mm in vivo, consistent with the lower end of ultrasound literature), expands primarily through the submucosa and muscularis mucosae, with the biopsy depth of 0.6 mm sampling only the mucosa.
Flier and colleagues (Front Med, 2020) identified two fibrosis hot spots in Crohn's resection specimens: the submucosa and the subserosa. The model treats the bowel wall as a six-layer structure (mucosa, muscularis mucosae, submucosa, inner and outer muscularis propria, subserosa) and solves a reaction-diffusion equation to estimate how far a luminally released drug can reach. The physics can be pictured as ink spreading through a wet sponge that is simultaneously being flushed with clean water: the balance between diffusion (spreading) and vascular clearance (washout) determines penetration depth. The governing equation is d/dx[D(x) dC/dx] minus k(x)(C minus C_blood) = 0, where D(x) is the local effective diffusion coefficient and k(x) combines vascular clearance and first-order metabolism. The clearance term uses Q = 0.25 ml/min/g for mucosal blood flow (Holm 1988; Granger 1980), an extraction ratio of 0.4 for small lipophilic molecules (Rowland and Tozer, textbook "Clinical Pharmacokinetics and Pharmacodynamics"), and a tissue water fraction of 0.70 (Boix 2005). Metabolism is modeled as a first-order sink based on ileal CYP3A4/5 expression at approximately 30 to 50% of hepatic levels (Paine 2006), contributing 20% of the mucosal vascular clearance rate in the mucosa and smaller fractions in deeper layers. This equation defines the steady-state ceiling: the maximum concentration achievable at each depth under continuous dosing.
The aqueous diffusivity of PALI-0008 was set at 5.5 x 10^-6 cm^2/s based on published values for PDE4 inhibitors of comparable molecular weight, cross-checked against the Stokes-Einstein equation (predicted 5.97 x 10^-6 cm^2/s, within 9%, using a hydrodynamic radius of 5.5 Å at 310 K). Both D(x) and k(x) vary continuously with sigmoid-blended layer boundaries. In established fibrosis, collagen deposition and vascular rarefaction depress both parameters, but k falls faster than D, so fibrotic tissue is paradoxically more permeable to drug in the model because reduced washout lets drug accumulate. Grid convergence was verified across N = 250 to 2000 nodes (depth variation less than 0.01 mm).
Under baseline fibrotic conditions, the steady-state therapeutic depth reaches 2.7 mm in normal ileum (99% of wall), 3.0 mm in the mild FSCD scenario (60%), and approximately 3.1 mm in both the moderate and severe FSCD scenarios (39% and 26% of wall, respectively). The depth plateaus near 3.1 mm because in severe disease, extreme submucosal fibrosis depresses diffusivity enough to partially offset the benefit of vascular rarefaction. These numbers represent theoretical upper bounds under idealized continuous dosing, not predictions of what any real patient would experience.
Fig. 2 shows the steady-state concentration profiles through the full wall thickness in each scenario. It is the clearest visualization of the central finding: drug concentration decays steeply with depth, and the fraction of wall reached shrinks as the wall thickens from normal to severe FSCD.
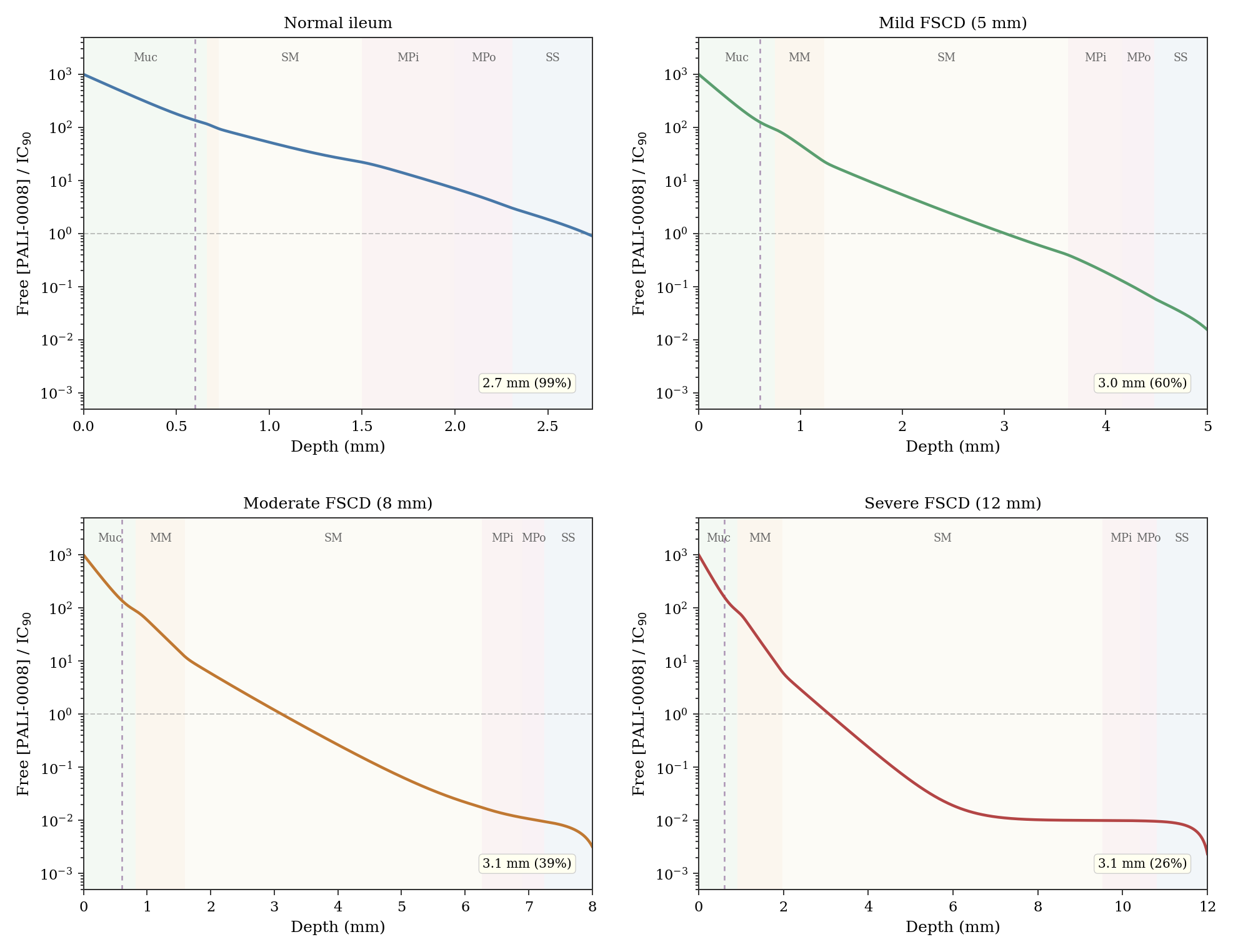
These steady-state depths assume purely fibrotic strictures with rarefied vasculature, which is the best case for penetration. But real strictures are rarely purely fibrotic; most contain zones of active inflammation with hyperemic neovasculature. This is arguably the most consequential assumption in the model. A sensitivity analysis (Fig. 3) shows that doubling vascular clearance (mild hyperemia) reduces the steady-state depth in the moderate FSCD scenario from 3.1 to 1.8 mm. At 3x baseline clearance the depth drops to 1.4 mm; at 5x (florid inflammation) only the mucosa is reliably treated. In a mixed fibro-inflammatory stricture, the realistic penetration could be substantially less than the baseline fibrotic numbers suggest. Notably, early strictures tend to be more actively inflamed than late-stage burned-out strictures, so the patients most suited to early intervention may have somewhat less favorable penetration profiles than the baseline fibrotic scenario models.
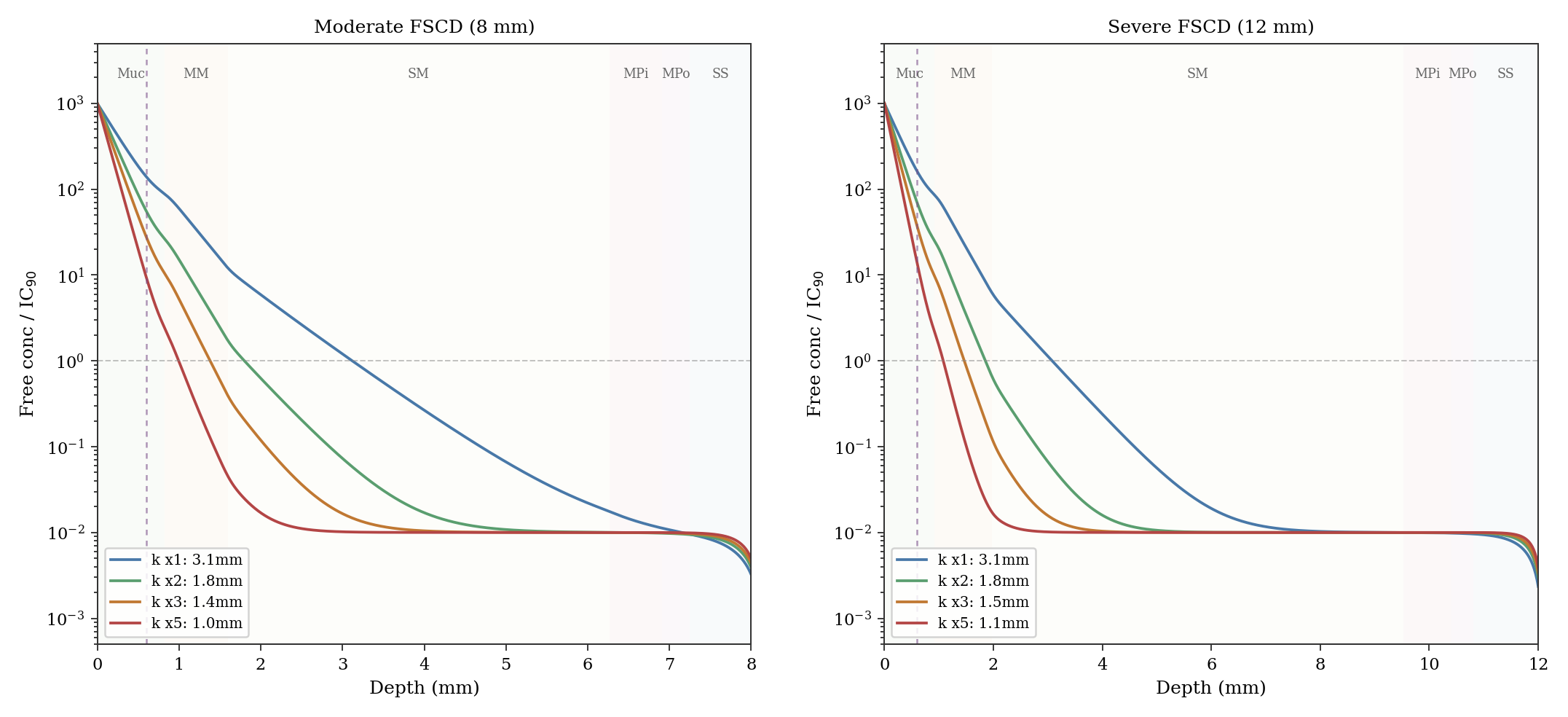
Reaching even the fibrotic ceiling takes time, governed by tissue binding. When drug arrives at a new region, the tissue absorbs it into proteins and lipid membranes. Only after local binding sites are substantially filled can free drug concentration rise enough to push the diffusion front deeper. The time-dependent model adds a retardation factor R(C): the ratio of total drug (free plus bound) to free drug, which varies with local concentration through a split-binding formulation. Non-specific hydrophobic partitioning into lipid membranes contributes a linear term (approximately 424), while specific PDE4 binding adds a saturable Langmuir term (approximately 20, with Kd of 10x IC90). At low concentrations R approaches 445; near the surface where drug is abundant and specific sites saturated, R falls to approximately 425. The saturable component represents roughly 4 to 5% of total partitioning and has negligible impact on penetration dynamics. A split-binding sensitivity analysis confirms this: varying the linear/specific ratio while maintaining the same total R produces virtually identical depth trajectories.
The model's own mucosal perfusion parameters imply a free-drug clearance half-life of about 5 minutes; compared to the observed 36-hour tissue half-life, this gives R of approximately 445 as the primary estimate. A more optimistic alternative of R approximately 301 has been used in earlier analyses, and both fall within a plausible range of 150 to 500 given the uncertainty in perfusion rates and tissue water fraction. Non-binding mechanisms such as continued prodrug conversion from residual luminal drug or slow mucus gel release could contribute to the observed 36-hour half-life, which would mean the true binding-driven R is somewhat lower than 445. The time-dependent equation R(C) times dC/dt = d/dx[D(x) dC/dx] minus k(x)(C minus C_blood) is solved using a Crank-Nicolson scheme (second-order in time), with the surface boundary condition cycling through an 8-hour dosing plateau followed by exponential decay with a half-life of approximately 2 hours. Surface concentration, decay rate, and boundary shape are all unsourced assumptions explored through sensitivity analysis. The 8-hour plateau may be optimistic for ileal delivery given typical 2-to-4-hour small bowel transit, though stricture-related stasis could prolong local exposure.
Under once-daily dosing, this tissue binding retards the advancing front during the dosing window but also acts as a reservoir during the 16-hour overnight interval. With R approximately 445 (the primary estimate), the front has reached 2.1 mm at Day 14 in the moderate FSCD scenario (66% of the theoretical steady state), with the biopsy zone maintaining trough concentrations at approximately 120 times IC90. With R approximately 21 (the minimum achievable under the split-binding parameterization, representing a hypothetical fast-binding scenario), the front reaches deeper faster but overnight troughs drop more steeply. Both scenarios maintain trough concentrations far above IC90 in the biopsy zone, explaining why the retardation factor does not affect biopsy pharmacodynamics. Fig. 4 shows how the therapeutic depth builds over days of dosing across all four scenarios.
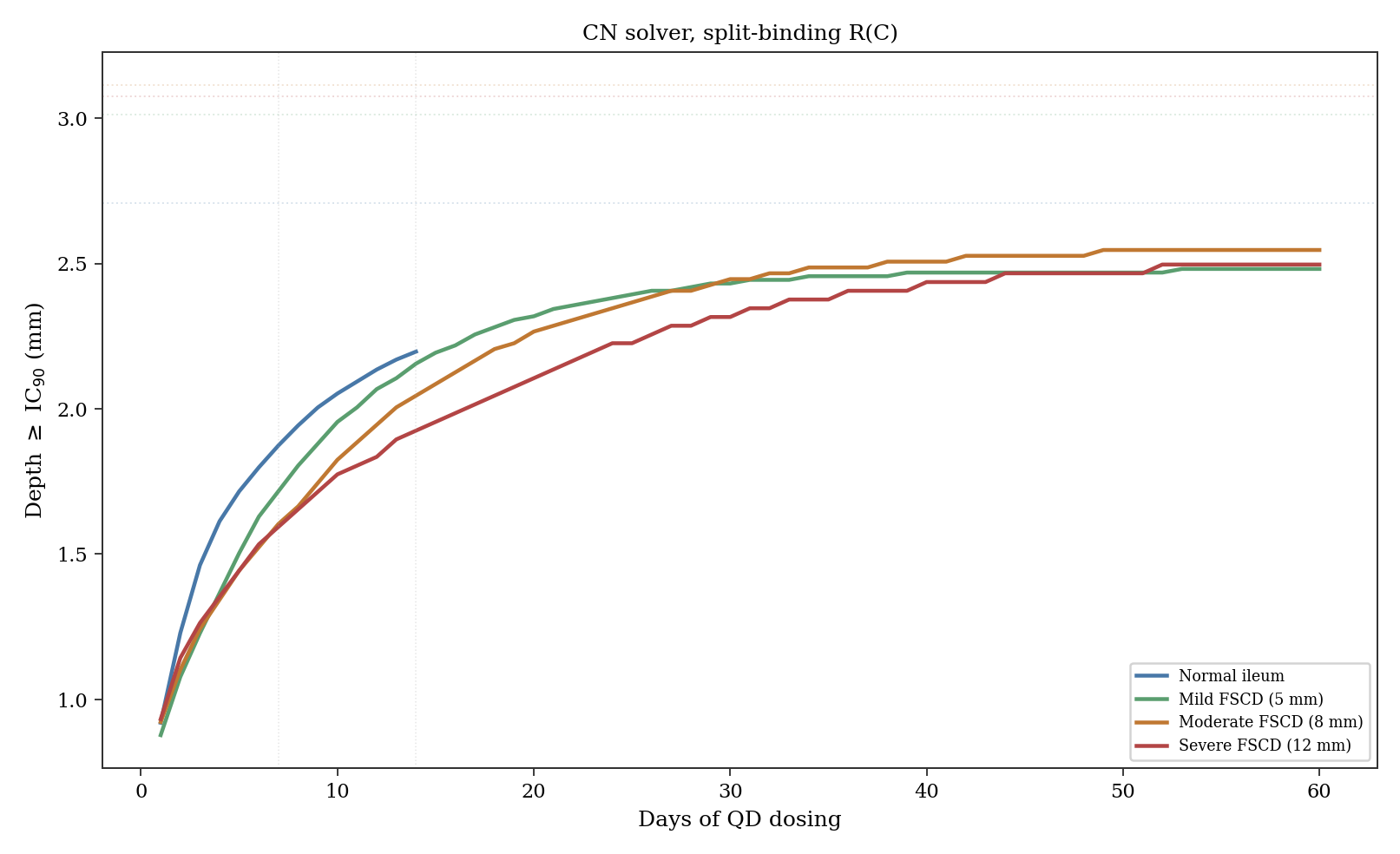
The biopsy zone (0 to 0.6 mm) achieves high concentrations quickly under any binding model. At Day 14 trough, total drug (free times local R) is roughly 50,000 times IC90 under R approximately 445 and much lower under fast-binding scenarios. The mucosal equilibration time constant (R/k, where k includes vascular clearance and metabolism) is about 43 hours under R approximately 445 and about 2 hours under R approximately 21. This explains why the Phase 1b UC results (7 days, BID) showed strong pharmacodynamic effects: the biopsy-accessible tissue was near steady state regardless of deep-tissue kinetics.
This rapid mucosal equilibration creates a problem for the proposed biopsy validation. Under R approximately 445, the biopsy zone reaches roughly 87% of steady state by Day 3 and plateaus by Day 7; under fast binding it reaches steady state within hours. Comparing Day 3 and Day 14 biopsies cannot discriminate fast from slow binding. The discrimination window is earlier: at 6 to 8 hours post-first-dose, R approximately 445 gives about 55% of steady state while fast binding is already at plateau, a nearly 2-fold difference detectable by LC-MS/MS. A single early biopsy at 6 to 8 hours post-first-dose would immediately constrain the effective retardation factor. Surface concentration follows logarithmic scaling: from 300 to 3,000 times IC90 in the moderate FSCD scenario, the steady-state depth moves from 2.4 to 3.8 mm.
A combined uncertainty analysis over 81 parameter combinations (three levels each for R, surface concentration, decay time, and vascular clearance multiplier) produces a Day 14 depth distribution with a median near 2 mm and a 5th-to-95th percentile range spanning approximately 1 to 3 mm. The biopsy zone remains saturated far above IC90 across every parameter combination tested, consistent with the Clopper-Pearson 95% lower bound of 54.9% derived from the five-of-five UC biopsy responses.
In the moderate FSCD scenario (8 mm wall), the Day 14 therapeutic depth of approximately 2.1 mm covers the mucosa, the expanded muscularis mucosae, and the proximal submucosa, but falls short of the deep submucosa and muscularis propria. In the severe FSCD scenario (12 mm wall), the same depth reaches an even smaller fraction. A surface reading would suggest PALI-2108 cannot treat transmural FSCD. But that reading assumes the goal is to treat established severe strictures from the lumen, and that framing may miss the more relevant clinical scenario.
Consider the trajectory of the disease. Strictures do not appear overnight. The progression from mucosal inflammation to transmural fibrosis occurs over years, driven by chronic TGF-beta/Smad signaling, myofibroblast activation, and extracellular matrix deposition. In early FSCD the bowel wall is perhaps 5 mm thick rather than 8 or 12. The submucosa begins at about 1.2 mm from the surface rather than 1.6 mm or more in advanced strictures. The model suggests that in mild FSCD, the steady-state depth of 3.0 mm would cover 60% of the wall, reaching well into the submucosal fibrotic zone. Even under transient Day 14 conditions, an estimated 2 mm of penetration in a 5 mm wall extends past the muscularis mucosae and into the submucosa, which is one of the two principal fibrotic zones identified by Flier et al.
This is where the anti-fibrotic mechanism becomes the argument. If PALI-2108 can reach the submucosa in early strictures and suppress fibrotic signaling at that stage, the wall may never thicken to the point where penetration becomes insufficient. The same drug that cannot treat an established 12 mm stricture might prevent that stricture from ever forming. This concept of intercepting Crohn's disease progression before strictures become irreversible has been a driving principle behind the STAR consortium's work on identifying "rapid progressors" who develop intestinal obstruction within 2 years of diagnosis. What the diffusion model adds is a quantitative framework for why timing matters specifically for a luminally delivered drug. Serosal-side fibrosis driven by creeping fat and mesenteric inflammation could remain beyond the reach of any luminally delivered drug.
There is supporting precedent from other organ systems. In idiopathic pulmonary fibrosis, nerandomilast slowed disease progression rather than reversing established fibrosis. The clinical value came from stabilization, not reversal. PALI-2108 does not need to dissolve existing strictures. It needs to halt the fibrotic cascade in tissue it can reach, before the wall outgrows the drug's penetration range. The fact that the biopsy zone shows trough concentrations exceeding 100 times IC90 under any binding scenario, and that the Phase 1b UC data confirmed downregulation of fibrotic gene signatures in that zone, suggests the anti-fibrotic machinery is being engaged where the drug is present. Whether that zone extends deeply enough in early FSCD to alter the natural history of the disease is the question the ongoing trial is designed to begin exploring.
Model limitations deserve emphasis, and there are many. This is a speculative exercise built on a stack of assumptions, each of which introduces uncertainty. The morphometric foundation (Zhang 2018) derives from ileal strictures and applies to the FSCD trial's ileal biopsies, but colonic FSCD sites would differ. The Phase 1a tissue pharmacokinetics come from colonic biopsies in healthy volunteers, not from ileal tissue in strictured patients; ileal and colonic tissue differ in vascularity, wall structure, and bacterial composition affecting prodrug activation. This last point deserves particular emphasis because the terminal ileum is the dominant stricture location in FSCD, involved in roughly 65 to 80% of cases either alone or with concurrent colonic disease (Freeman 2007).
Normal distal ileum harbors only 10⁷ to 10⁸ CFU/mL versus 10¹¹ to 10¹² in the colon (Kastl 2020), a three-to-four order-of-magnitude gap, and ileal β-glucuronidase activity is normally low (Roberton 1982). However, stricture-related luminal stasis promotes bacterial overgrowth; SIBO prevalence in Crohn's disease is estimated at 25 to 88%, with the fibrostenotic phenotype carrying a significantly elevated risk (Feng 2024; Klaus 2009). Most ileal strictures sit near the ileocecal valve where cecal reflux could elevate local enzyme activity, and patients with prior ileocecal resection lack this anatomic barrier entirely, resulting in a terminal ileum microbiome resembling that of the colon (StatPearls SIBO). Whether these compensatory factors close the gap sufficiently is unmeasured and represents the gating pharmacokinetic uncertainty for the FSCD program: if the prodrug cannot generate adequate PALI-0008 at ileal stricture sites, penetration depth becomes moot. The FSCD trial's paired ileal biopsies (Palisade Bio, Oct 2025), which include tissue PK and transcriptomic endpoints, should begin to address this question.
The model assumes hypertrophic strictures, but Liu et al. (2022) showed a subset are constrictive, with reduced circumference and little mural thickening; drug penetration in those strictures might differ unpredictably. The layer-specific formalin shrinkage corrections (1.25x to 1.54x) sit within the published range of 1.1 to 1.6x per dimension. The baseline assumes fibrotic vascular rarefaction (best case for penetration); as shown in Fig. 3, even mild hyperemia nearly halves depth. The split-binding proportions (424 linear, 20 saturable) are estimated from class-level PDE4 pharmacology, not direct tissue binding studies. The total binding ratio of approximately 444, derived from the tissue/clearance half-life ratio, has a plausible range of 150 to 500. More optimistic estimates around 300 would produce somewhat faster front advance and slightly deeper Day 14 penetration, but the qualitative picture remains the same.
Additional technical limitations include: diffusivity derived from porosity principles rather than direct measurement; vascular clearance in the expanded muscularis mucosae assumed to scale as the square root of fold-expansion (unmeasured); a constant extraction ratio of 0.4 across all layers, ignoring dependence on local free drug fraction and capillary transit time; and a uniform binding ratio across layers when different tissue types would have different partition coefficients. The model is one-dimensional, does not model prodrug conversion kinetics or the unstirred water layer, and ignores lymphatic clearance, venous redistribution, and paracrine signaling from PDE4 inhibition. None of these limitations individually invalidate the model, but together they mean the outputs should be understood as order-of-magnitude estimates, not precise predictions.
The Phase 1b FSCD study, dosing since October 2025, enrolls 6 to 12 patients for 14 days of once-daily PALI-2108 with paired ileal biopsies, single-nucleus RNA sequencing, and exploratory intestinal ultrasound. The model suggests 14 days is sufficient to saturate the biopsy zone under any binding hypothesis. The dominant linear partition means the diffusion front reaches roughly two-thirds of steady-state depth at Day 14, with practical equilibrium near 3 mm only by Day 40 to 60. If the trial could incorporate an early biopsy at 6 to 8 hours post-first-dose, the resulting concentration would immediately reveal whether effective mucosal retardation is closer to 21 or 445, determining whether the deep-tissue timeline is measured in days or weeks.
The science behind PALI-2108 is sound, and the case for early intervention in FSCD deserves serious attention. But readers should be clear-eyed about what this analysis is and is not. It is a theoretical exploration that identifies plausible ranges and highlights which variables matter most. It is not a prediction of clinical outcomes. Under the model's central assumptions (R approximately 445, QD dosing, baseline fibrotic conditions), Day 14 therapeutic depth reaches approximately 2.1 mm in the moderate FSCD scenario. Hyperemia could reduce this by 40 to 60%. For advanced strictures exceeding 8 to 10 mm, the drug likely cannot reach the deep fibrosis driving obstruction from the lumen alone.
But the more important finding may be that the model did not rule out meaningful submucosal penetration, even under conservative structural choices including one-dimensional geometry, single-dose pharmacokinetic extrapolation, and purely fibrotic baseline conditions. Several of these choices were deliberately conservative, and the fact that the drug still reaches the proximal submucosal fibrotic zone in early and moderate disease is itself notable. The model's answer to whether PALI-0008 could theoretically reach the deeper intestinal wall where fibrosis occurs is a qualified yes, dependent on early intervention and the degree of inflammatory hyperemia.
It should be noted, though, that we assumed based on class-level PDE4 pharmacology that PALI-0008 can affect fibrosis in the intestinal wall; this has not been directly demonstrated for this compound. The ongoing FSCD trial, together with future controlled studies, will determine whether the theoretical promise holds up in actual patients. In a disease where no approved anti-fibrotic therapy exists and surgery rates have barely improved in two decades of biologic therapies, a drug that could intercept fibrotic progression at an early stage would represent a genuine shift in the treatment paradigm.
Follow me on X for frequent updates (@chaotropy).
Legal Information and Disclosures
General Disclaimer & No Financial Advice: The content of this article is for informational and educational purposes only. It represents the personal opinions of the author as of the date of publication and may change without notice. The author is not a registered investment advisor or financial analyst. This content is not intended to be, and shall not be construed as, financial, legal, tax, or investment advice. It does not constitute a personal recommendation or an assessment of suitability for any specific investor. Readers should conduct their own independent due diligence and consult with a certified financial professional before making any investment decisions.
Medical Disclaimer: Although the author possesses a medical background, the information presented here regarding medical technologies, clinical trials, or pharmaceutical mechanisms is strictly for the purpose of educational discussion and general commentary regarding the underlying science. It does not constitute medical advice, a diagnosis, or a treatment recommendation, nor does it establish a physician-patient relationship. Readers should never disregard professional medical advice or delay in seeking it because of something read on this website. Always consult a qualified healthcare provider regarding any medical condition.
Computational Model Disclaimer: The reaction-diffusion model presented in this article is a speculative theoretical exercise. It relies on estimated parameters, published morphometric data from resection specimens, and class-level pharmacological assumptions rather than direct measurements of PALI-0008 diffusivity, binding, or clearance in strictured human tissue. Many inputs (including tissue binding ratios, vascular clearance rates, layer-specific diffusivities, and the degree of inflammatory hyperemia) are approximated from literature on related compounds or inferred indirectly. The model's outputs should be understood as order-of-magnitude estimates illustrating relative trends, not as quantitative predictions of drug penetration in any individual patient or clinical trial. Reproducing the model's numerical outputs does not constitute independent validation of its assumptions or conclusions.
Accuracy and Third-Party Data: Market trends, clinical trial data, and performance metrics referenced in this article are sourced from independent third parties. While the author believes these sources to be reliable, the completeness, timeliness, or correctness of this data cannot be guaranteed. The author assumes no liability for errors, omissions, or the results obtained from the use of this information.
Disclosure of Interest: The author holds a beneficial long position in Palisade Bio, Inc. (NASDAQ: PALI). The author reserves the right to buy or sell these securities at any time without further notice. The author receives no direct compensation for the production of this content and maintains no business relationship with the companies mentioned.
Forward-Looking Statements & Risk: This article contains forward-looking statements regarding regulatory outcomes (such as FDA decisions), clinical results, and market potential. These statements are predictions based on current expectations and are subject to significant risks and uncertainties. Investing in biotechnology and pharmaceutical securities involves a high degree of risk, including the potential for total loss of principal. Past performance is not indicative of future results.
Copyright: All content is the property of the author. This article may not be copied, reproduced, or published, in whole or in part, without the author's prior written consent.